Arrange the Compounds From Highest to Lowest Boiling Point.
The four compounds are alkanes and nonpolar so London dispersion forces are the only important intermolecular forces. Specific heat of liquid 374 Jg K.

Solved Arrange The Compounds From The Highest Boiling Point Chegg Com
ΔHvap 351 Jg.

. Will melt rather than sublime at STP e. NCERT Exemplar Class 11 Chemistry Chapter 5 States of Matter are part of NCERT Exemplar Class 11 Chemistry. Before finding this i was looking through my class notes and textbook trying to find out what made a difference in a molecules boiling point and i couldnt find a good answerusing this page i was able to answer a question on my assignment.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions. CH 3 CH 2 OH e. Boiling point 345 C.
The I effect is the highest in p-tolualdehyde because of the presence of the electron-donating -CH 3 group and the lowest in p-nitrobezaldehyde because of the presence of the electron-withdrawing -NO 2 group. By thinking about noncovalent intermolecular interactions we can also predict relative melting points. A substance whose triple point occurs at 222K and 393 atm _____.
Rank the following compounds in order from lowest boiling point to highest boiling point. Consequently we can easily say that propane having the smallest molecular mass will have the lowest boiling point. Arrange the following compounds in order of increasing attraction between their ions.
Based on their structures rank phenol benzene benzaldehyde and benzoic acid in terms of lowest to highest boiling point. This is very helpful boiling point started to make sense already by that list at the beginning. CH 3 OCH 3 d.
Will not have a critical point d. Compounds are defined as substances composed of two or more different elements that are chemically. The boiling points of these aqueous solutions can then be compared based on the concentrations of each solution.
Im going to study the printable sheet. Here we have given NCERT Exemplar Class 11 Chemistry Chapter 5 States of Matter. Which of the following polar compounds is likely to have the highest boiling point.
NCERT Exemplar Class 11 Chemistry Chapter 5 States of Matter Multiple Choice Questions Single Correct Answer Type Q1. Draw the lewis structure identify the shape state whether it is polar or nonpolar and identify the one with lowest boiling point. Similarly since n-pentane has the largest molecular mass the boiling point will be the highest.
Stronger intermolecular interactions result in a higher melting point. According to Table PageIndex1 the molal boiling point elevation constant for water is 051Cm. The larger the molecule the larger the dispersion forces and the higher the boiling point.
Will sublime rather than melt at STP c. A person living in Shimla. Will have a critical point of 211K and 293 atm b.
Specific heat of gas 235 Jg K A 101 kJ B 131 kJ C 161 kJ D 486 kJ 38. Sodium nitrate highest boiling point glucose sucrose lowest boiling point Colligative properties depend on the total number of solute where the type of solute particles can be ignored. Arrange the following compounds from highest boiling point to lowest boiling point and explain your answer on the basis of whether the substance is.
The substance with the weakest forces will have the lowest boiling point. Which of the following liquids would have the lowest viscosity factoring in both the impact of the substance and the. CH3CH2CH3 propane or CH3CH2CH2CH3 butane has the highest melting point because they both have the same IMF London Dispersion however butane has the higher molecular weight.
Will have a critical point of 233K and 293 atm. H 2 CO b. Determine the intermolecular forces in the compounds and then arrange the compounds according to the strength of those forces.
All of these compounds are nonpolar and only have London dispersion forces. As the molecular mass of the compound increases the forces between them get more robust. All of the same principles apply.
Thus a 100 m aqueous solution of a nonvolatile molecular solute such as glucose or sucrose will have an increase in boiling point of 051C to give a boiling point of 10051C at 100 atm. The ordering from lowest to highest boiling point is therefore C 2 H 6 C 3 H 8 C 4 H 10. Examine the following phase diagram and identify the feature represented by point A.

Solved Arrange The Following Compounds In Order From Highest Chegg Com
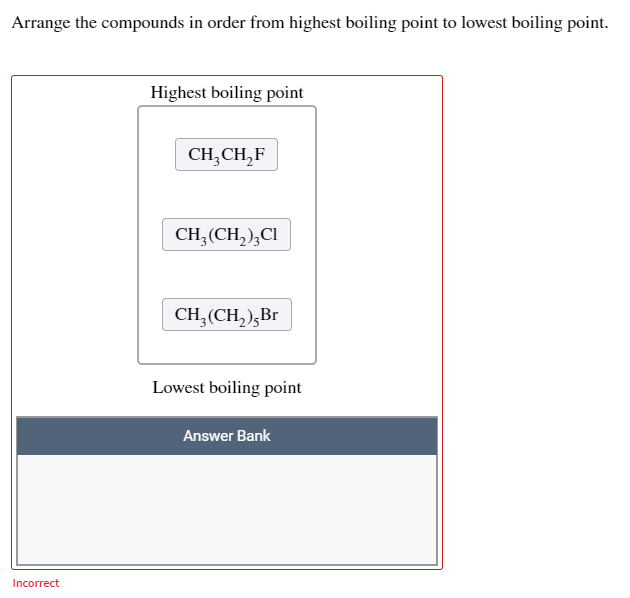
Solved Arrange The Compounds In Order From Highest Boiling Chegg Com

Chem 3102 Sapling Week 8 Exp 3 3 A B Structural Effects Of Boiling Point And Refractive Index Unknown Liquid Flashcards Quizlet

Answered Arrange The Following Compounds In Bartleby
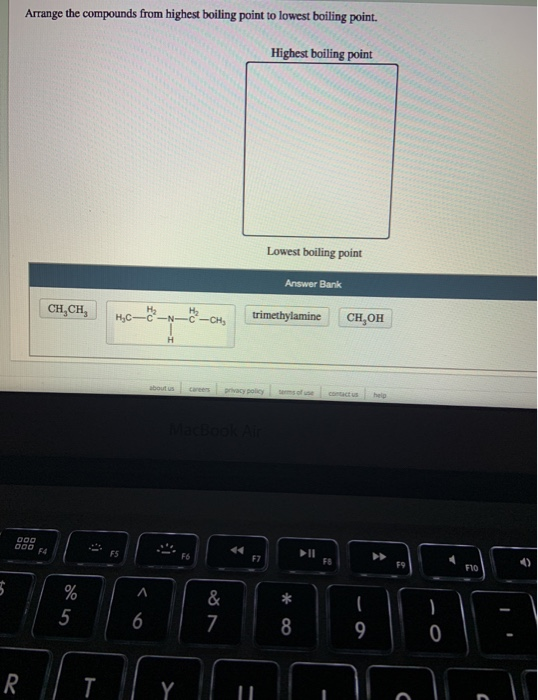
Solved Arrange The Compounds From Highest Boiling Point To Chegg Com

Solved Arrange The Compounds By Increasing Boiling Point Lowest Boiling Point Ch C Ch Ch Cooh Ch Ch Coz Ch Ch Ch Chz Ch Ch Ch Ch C Onhz Highest Boiling Point
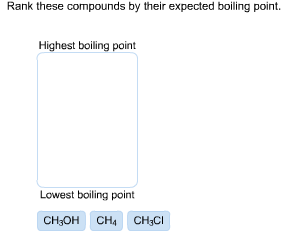
Rank These Compounds By Their Expected Boiling Point Highest Boiling Point Lowest Boiling Point Ch3oh Home Work Help Learn Cbse Forum
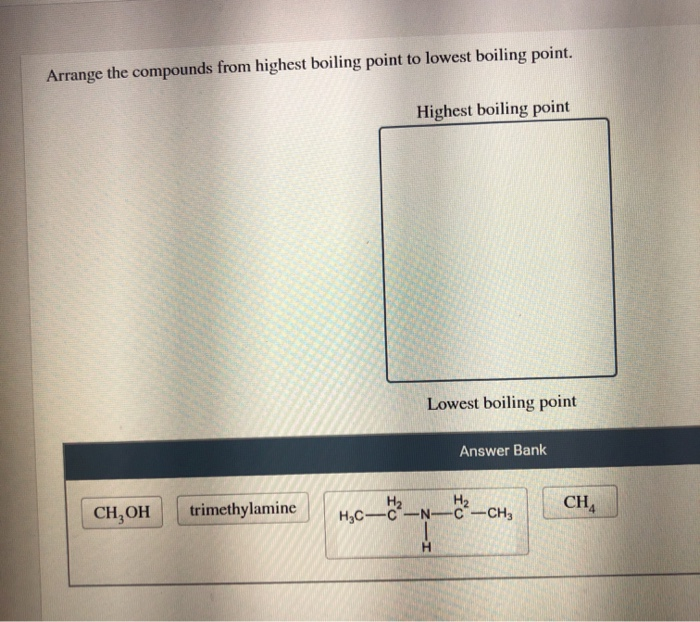
Solved Arrange The Compounds From Highest Boiling Point To Chegg Com

Solved Arrange The Compounds In Order From Highest To Lowest Chegg Com

Arrange The Following Compounds In Order Of Increasing Boiling Point Putting The Compound With The Lowest Boiling Point First Explain Your Answer Study Com
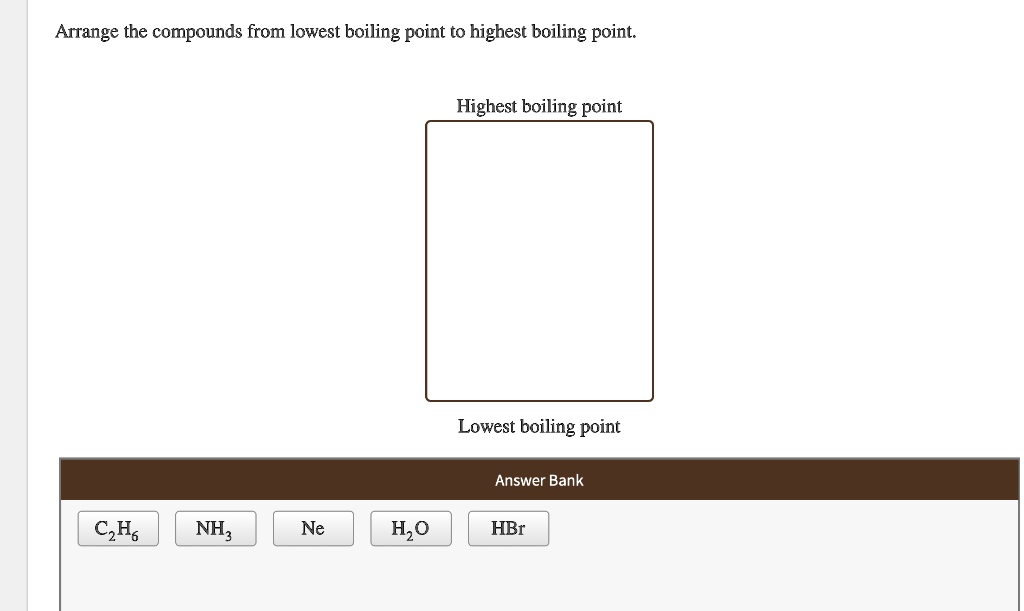
Solved Arrange The Compounds From Lowest Boiling Point To Highest Boiling Point Highest Boiling Point Lowest Boiling Point Answer Bank Czh Ne Hzo Hbr Nh

Solved The Due Date Tor This Activity Has Passed Assignment Score 35 690 Resources Glve Upl Hint Questlon 21 0f 25 Arrange The Compounds Trom Highest Boiling Point To Lowest Boiling 5 Pomnt Highest
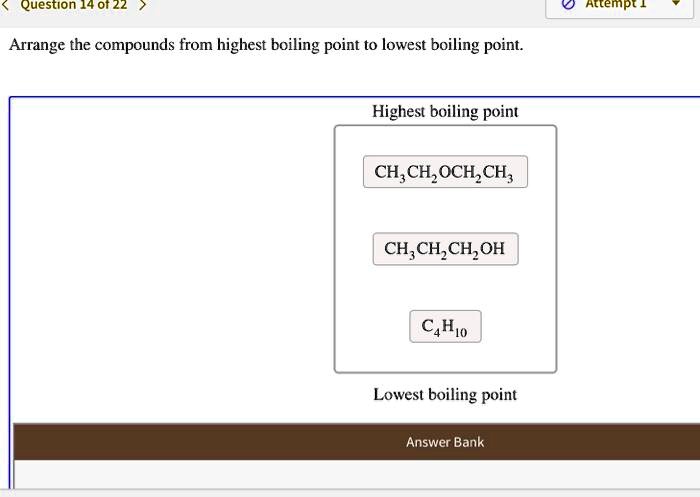
Solved Question 14 01 22 Attempt 1 Arrange The Compounds From Highest Boiling Point To Lowest Boiling Point Highest Boiling Point Ch Ch Och Ch Ch C Ch Jch Oh C4hjo Lowest Boiling Point Answer Bank

Solved Question 16 Of 21 Arrange The Compounds From Chegg Com

Answered Arrange The Compounds In Order From Bartleby
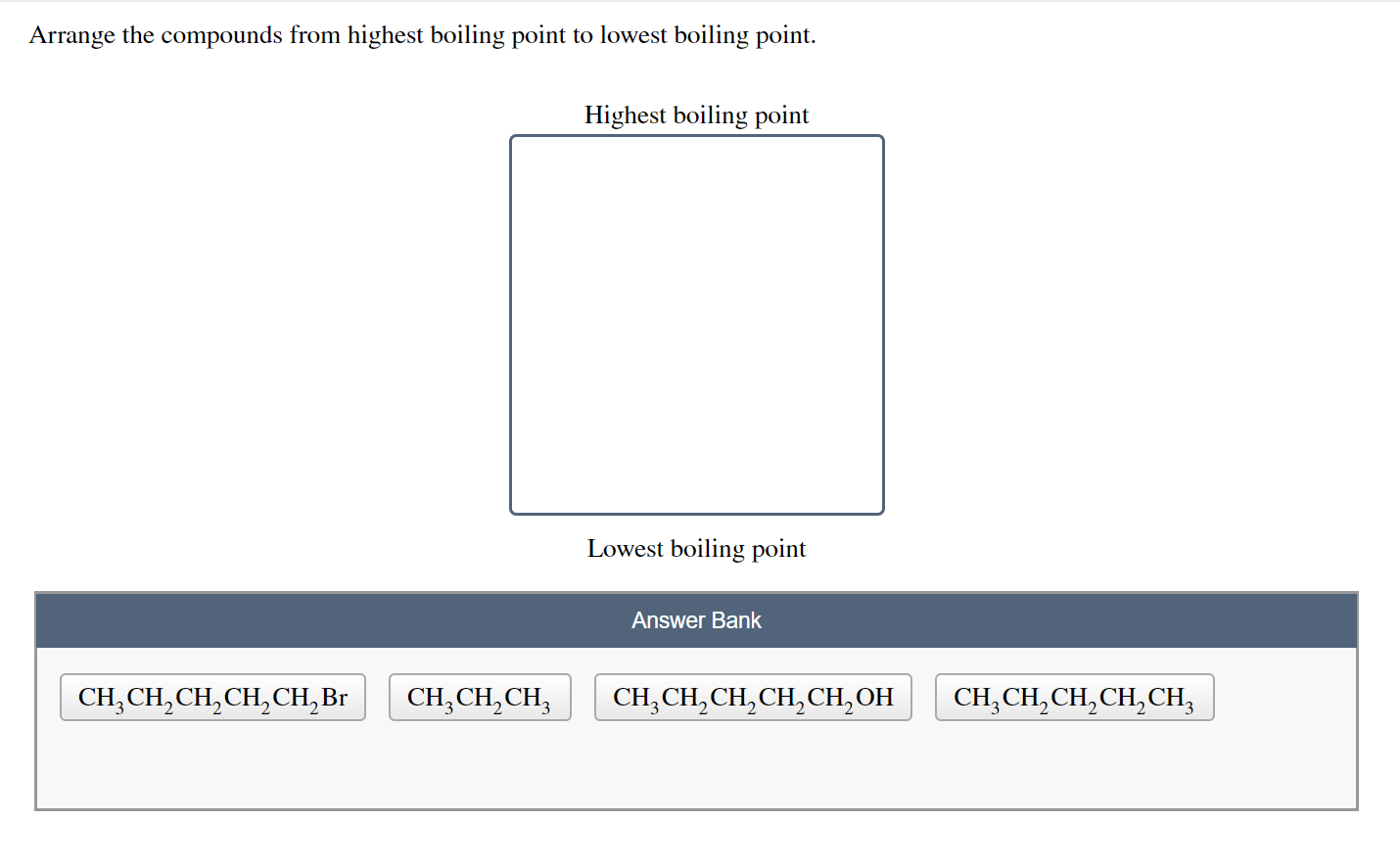
Solved Arrange The Compounds From Highest Boiling Point To Chegg Com

Solved Arrange The Compounds From Lowest Boiling Point To Chegg Com

Answered Arrange The Compounds From Highest Bartleby
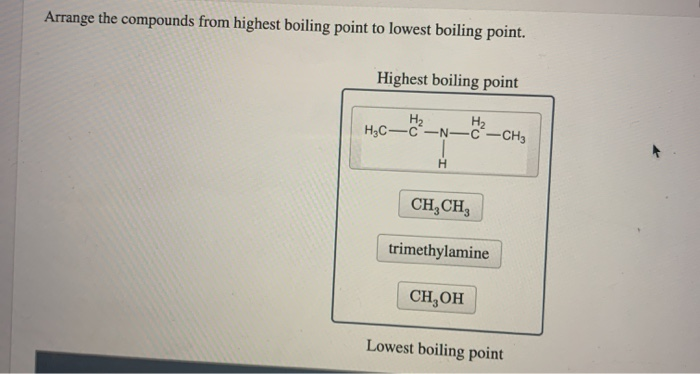
Solved Arrange The Compounds From Highest Boiling Point To Chegg Com
Comments
Post a Comment